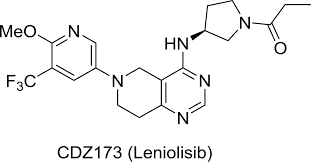
Introduction
Leniolisib has emerged as a groundbreaking treatment for Apoptosis-Specific Precursor T-cell Immunodeficiency Syndrome (APDS), a rare genetic disorder that affects the immune system. This condition is characterised by recurrent infections, increased susceptibility to autoimmunity, and difficulty in responding to vaccines. The significance of leniolisib lies not only in its potential to improve the quality of life for patients suffering from APDS but also in the innovative approach it takes to manage immune deficiencies.
The Mechanism of Leniolisib
Leniolisib is a selective inhibitor of the phosphoinositide 3-kinase delta (PI3Kδ) pathway. This pathway plays a critical role in the functioning of immune cells. By targeting this pathway, leniolisib effectively modulates immune responses, thereby restoring normal immune function in patients with APDS. Clinical studies have shown that leniolisib can significantly reduce the frequency of infections and improve overall health in affected individuals.
Recent Developments
In September 2023, leniolisib received marketing authorisation from the European Commission for the treatment of adults and children aged 12 years and older with APDS. This approval followed encouraging results from Phase 3 clinical trials, which demonstrated the drug’s efficacy and safety. Patients experienced a marked decrease in infection rates and improvements in quality of life metrics when treated with leniolisib. Regulatory agencies in other regions, including the FDA in the United States, are also considering applications for leniolisib, indicating a growing recognition of its importance.
Significance for Patients and Healthcare Providers
The availability of leniolisib offers new hope for APDS patients, who have previously had limited treatment options. This medication provides a targeted approach to managing immune deficiencies and may lessen the burden on healthcare systems by reducing hospitalisations associated with recurrent infections. For healthcare providers, leniolisib represents an advancement in personalised medicine, enabling more tailored treatments for patients based on their specific genetic and immunological profiles.
Conclusion
The introduction of leniolisib marks a significant breakthrough in the management of APDS and highlights the importance of continued research in rare genetic disorders. As more patients gain access to this treatment, it is essential to monitor its long-term effects and refine treatment protocols. The future looks promising for individuals with APDS, as leniolisib not only presents an effective treatment option but also paves the way for further advancements in precision medicine, aiming to improve the lives of those affected by similar immune deficiencies.
You may also like

Understanding Melatonin: Uses, Benefits, and Importance

Understanding the Importance of Bones for Our Health

Understanding Omeprazole: Benefits and Considerations
SEARCH
LAST NEWS
- Remembering Wendy Richard: The Promise to Co-Star Natalie Cassidy
- How Did Anglian Water Achieve an ‘Essentials’ Rating for Mental Health Accessibility?
- Shai Hope Leads West Indies in T20 World Cup Clash Against South Africa
- What We Know About Weston McKennie: Future at Juventus and Past at Leeds
- What We Know About the Upcoming Live Nation Antitrust Trial