Introduction
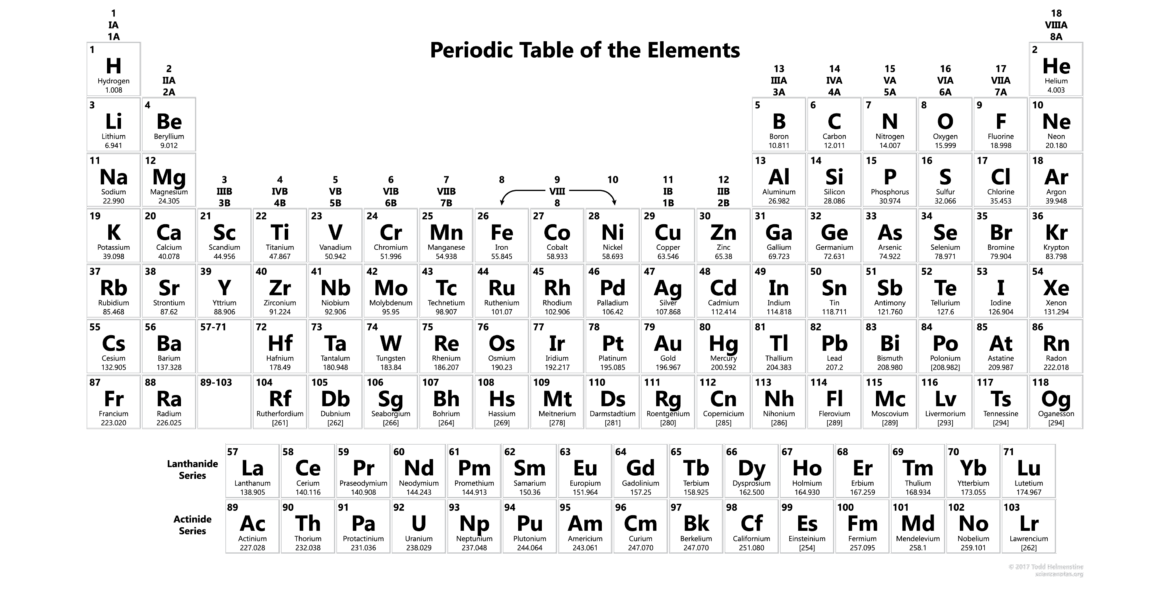
The periodic table of elements serves as a fundamental tool in chemistry, providing an organised framework for understanding the relationships among various chemical elements. Formulated in the 19th century, the table arranges elements in order of increasing atomic number, revealing patterns in their properties and behaviours. Its relevance extends beyond chemistry, impacting fields such as physics, biology, and environmental science. With the ongoing exploration of new elements, the periodic table continues to evolve, holding crucial implications for scientific advancement.
Structure of the Periodic Table
The periodic table comprises rows called periods and columns known as groups. There are currently 118 confirmed elements, each represented by a unique symbol. Elements in the same group share common properties; for example, group 1 (alkali metals) elements like lithium, sodium, and potassium are highly reactive, particularly with water. Conversely, noble gases found in group 18 are notable for their lack of reactivity.
The table is also divided into blocks based on electron configurations: the s-block, p-block, d-block, and f-block, further illustrating the behaviour of electrons and the resulting chemical characteristics. Recent additions, such as the discovery of new synthetic elements, highlight the table’s dynamic nature.
Recent Developments
As of late 2023, researchers continue to investigate and synthesise new elements that could be added to the periodic table, pushing the boundaries of what we know about matter. The pursuit of these superheavy elements provides insights into atomic stability and chemical behaviour under extreme conditions. Additionally, advancements in quantum physics and computational chemistry are providing deeper understanding of how elements interact and bond.
Conclusion
In summary, the periodic table is not merely a static arrangement of elements but a vibrant representation of our understanding of the natural world. Its importance resonates across multiple scientific fields. As researchers make strides in discovering new elements and understanding existing ones, the periodic table will likely expand and evolve, inviting future generations to explore its depths. This ongoing journey into the realm of chemistry underscores the significance of the periodic table as a cornerstone of scientific education and innovation.
You may also like

Is Lisa Riley Pregnant? What We Know So Far

The Eden Project: A Beacon of Sustainability and Education