Introduction to Group 7 Elements
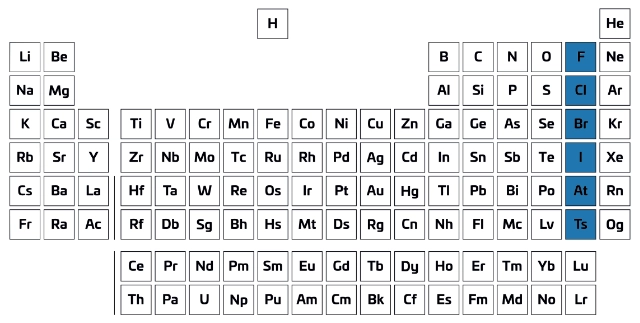
Group 7 of the periodic table, known as the halogens, includes five elements: fluorine, chlorine, bromine, iodine, and astatine. These elements are integral to a variety of chemical reactions and have significant applications in everyday life. Understanding their properties and behaviours is crucial for both students and professionals in the field of chemistry.
Properties of Group 7 Elements
The halogens are non-metal elements that are characterised by their high electronegativity and reactivity. Fluorine, the lightest halogen, is the most reactive. As one moves down the group, the reactivity decreases, with astatine being the least reactive. Fluorine is a gas at room temperature, chlorine is also a gas, bromine is a liquid, and iodine is a solid. This trend in physical state reflects their molecular weight and intermolecular forces, showcasing the diversity within the group.
Applications of Halogens
The halogens have numerous applications. For instance, chlorine is widely used in water treatment and disinfection, while fluorine is essential in toothpaste to prevent cavities. Bromine is employed in flame retardants and various pharmaceuticals, and iodine is crucial in medical antiseptics. Moreover, these elements play a pivotal role in other industries such as agriculture, where they are used in pesticides.
Environmental and Health Impacts
Despite their beneficial uses, halogens pose certain environmental and health risks. Chlorinated compounds can form harmful by-products during water treatment, and fluorinated gases contribute to global warming. Therefore, it is essential to handle these elements with care and assess their environmental impact during industrial processes.
Conclusion
Group 7 elements, known as halogens, are vital in both chemical processes and daily life applications. Their reactivity and compound formation capabilities make them incredibly useful, but also require careful handling due to potential health and environmental risks. As science advances, further research into safer alternatives and innovative applications continues to evolve, ensuring that the significance of Group 7 in chemistry and everyday life remains relevant.
You may also like

Unveiling the Mysteries of Aliens: Recent Discoveries

Recent Discoveries about Comets by NASA